Recently, an important research progress greatly stimulated the field of electrochemical energy storage. A joint research team from the University of Illinois at Chicago (UIC), Argonne National Laboratory, and California State University (Northridge) at Nature Articles published in the magazine--
Successfully made a lithium-air battery that can be cycled more than 700 times in an air-like atmosphere, breaking the previous limitation that lithium-air batteries can only use pure oxygen and having a short cycle life, allowing people to see that this has a very high theoretical energy. Density batteries replace existing lithium ions, breaking the potential of electric vehicle mileage bottlenecks.
What is a lithium-air battery? What is the difference between lithium air battery and lithium ion battery? Why is this breakthrough in lithium-air batteries important? This starts with the question of why lithium ion batteries have low energy density.
Lithium-ion batteries are by far the most successful rechargeable batteries. It is called "lithium-ion battery" because in the battery, regardless of charge or discharge, lithium ions (Li+) are shuttled back and forth between the two electrodes to form a current. Lithium ions need to be "embedded" on their surface when they reach the electrode and "deintercalated" when they leave. In order to ensure a good "embedded-deintercalated" reaction, the anode of a lithium ion battery is usually graphite, and the cathode is usually a lithium compound. For example, in the cathode of the most popular “Sanyuan Li†battery, in addition to lithium, nickel, cobalt, and manganese are also required to constitute the compound LiNi0.3Co0.3Mn0.3O2. And nickel, cobalt and manganese are much heavier than lithium.
Therefore, in a lithium-ion battery, although only one lithium ion with a relative atomic mass of 3 (a relative one is one twelfth of the mass of a carbon atom) can carry one unit of charge, its cathode is There is also a need for nickel, cobalt, manganese, iron, phosphorus, carbon, and other atomic constituent compounds that are much heavier than lithium to "accommodate" this lithium ion together. As a result, for the positive charge of this unit, only a “mock†with a relative molecular mass of approximately 100 may be provided only at the cathode. Coupled with the weight of the anode and other materials and structures, the energy density of a lithium-ion battery has not been able to do so. This is why, for an electric vehicle carrying half a ton of lithium-ion batteries, the cruising range is far less than the average car with just a few tens of liters of gasoline.
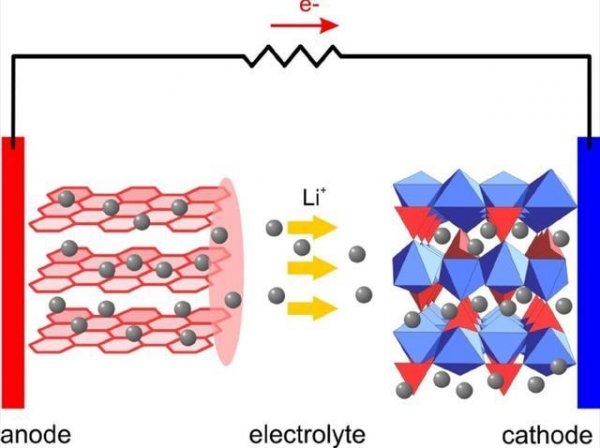
In a lithium-ion battery, in order to stably "store" lithium ions (grey spheres in the figure) carrying charges, a large number of other structures are required, such as lithium compounds (blue, red steric structures) and graphite ( The red layered structure), the relative atomic mass of these elements are far greater than that of lithium, and the energy density of lithium ion batteries is always limited. In an ideal lithium-air battery, these elements are not needed, just lithium metal and oxygen in the air!
Lithium-air batteries are different. Unlike lithium-ion batteries, which require lithium compounds and graphite electrodes, lithium-air batteries can directly use lithium metal (Li) and oxygen (O2) in the air as electrodes. In the most ideal case, when the battery is discharged, lithium oxide (Li2O2) is generated from oxygen-oxygenated lithium to generate electric current in the external circuit, and lithium peroxide and lithium oxide are decomposed by lithium peroxide during charging. The whole process does not require the participation of other elements with a larger mass, and the cathode can even directly use air with negligible weight and cost!
As a result, lithium-air batteries can achieve much higher energy densities than lithium-ion batteries. In fact, because lithium is the metal element with the lightest relative atomic mass in the periodic table, and oxygen comes from the air, lithium-air batteries have the highest theoretical energy density in electrochemical cells—in other words, the mass of lithium. Air batteries can store and release more energy than all other electrochemical energy storage media.
The non-liquid lithium-air battery has a theoretical energy density of 12 kWh/kg, which is 5 to 10 times that of existing lithium-ion batteries and is almost comparable to about 13 kWh/kg of gasoline. If the lithium-air battery can eventually reach the market, electric vehicles will also have the same cruising range as gasoline vehicles, which will completely break the bottleneck of cruising range caused by the low energy density of lithium-ion batteries, which is important for the future development of clean energy. The significance.
However, these are all theoretical analyses. It is not an easy task to achieve such an ideal situation.
Prior to this, lithium-air batteries, which could be called air cathodes, all depended on pure oxygen. This is because, in addition to oxygen, nitrogen, carbon dioxide, and water vapor in the air also participate in the reaction, making this process extremely complex. The oxidation of the anodic lithium, and the reaction of the cathode lithium ions with carbon dioxide and water vapor in the air generate undesirable by-products.
Because of other chemical reactions on the electrodes, electrolytes, and the chemical properties of metal lithium and oxygen are more active, the cycle life of lithium-air batteries has also been very short. In addition, the pure oxygen environment requires that the lithium air must be equipped with an oxygen storage device when used, such as a huge oxygen cylinder, which allows the high energy density of the lithium air battery to be directly amortized by the large and heavy oxygen storage tank, and the battery capacity It also depends on the capacity of the oxygen cylinder. What's more, if you want to use a lithium-air battery in an electric vehicle, oxygen cylinders will increase the safety risk in addition to a significant increase in weight.
In fact, if it is not because of the above defects, lithium-ion batteries will not be close to using complex electrodes. Because the lithium-air battery directly using lithium metal as the electrode cannot directly obtain the required oxygen in the air, some scientists even simply call the lithium-air battery a “lithium-oxygen batteryâ€.
After many years of development, these problems have always been shrouded in the head of lithium-air batteries, not to mention competition with lithium-ion batteries. Until this breakthrough of the University of Illinois at Chicago, Argonne National Laboratory, and California State University Northridge, it only brought hope for this excellent performance only in theory.
If you want to solve the fatal defects of lithium-air batteries, you must find ways to prevent the various chemical substances in the air - nitrogen, carbon dioxide, water vapor and other components involved in side reactions. These side reactions can have an effect on the electrodes, lithium ions, and electrolytes, producing unwanted by-products. Researchers conducted in-depth research on this issue using computer simulations (density functional analysis) and experimental methods. Finally, they found an answer: Add a protective layer on the lithium metal electrode.
The core of this technology is that at the anode, they add a dense protective coating of lithium Carbonate /carbon (LiCO3/C) to lithium metal.
The coating process is unusually simple: Lithium metal and carbon dioxide can be completed by direct chemical reaction on the surface of the electrode through 10 cycles of charge and discharge. Lithium carbonate blocks entry of compounds other than lithium ions, protecting the anode from damage by other components than oxygen in the air. In the atmosphere, lithium carbonate does not react spontaneously with the water vapor in the air, so this protective layer will not participate in the chemical reaction of the battery and will not be destroyed. Under the protection of the coating, the lithium retention rate of single cycle is as high as 99.97%, which is much better than the lithium air battery without coating.

Figure 丨 dense anode protection layer (scale bar: the length of the green line in the figure is 1 μm)

Figure is the molecular oxygen that is passing through the Li2CO3 coating
To test the performance of this battery, the researchers used a molybdenum disulfide (MoS2) nanosheet previously reported by other studies as a cathode and used 1-ethyl-3-methylimidazolium tetrafluoroborate ( A mixture of 1-ethyl-3-methylimidazolium tetrafluoroborate (EMIM-BF4) and Dimethyl sulfoxide (DMSO) was used as the electrolyte. The anode, cathode, and electrolyte work together and the lithium air battery is placed in a simulated air environment - 79% nitrogen, 21% oxygen, 500ppm carbon dioxide, and 45% relative humidity at a temperature of 25C. .
After testing, the lithium air battery did not show any failure after 700 cycles of charge and discharge. This achievement exceeds many people's expectations, and has even reached the cycle life of some mature commercial batteries (such as lead-acid batteries).
The research team therefore concluded that "protected lithium anodes, electrolyte mixtures, and high-performance air cathodes that work together under simulated air conditions can effectively increase the number of cycles in lithium-air batteries."
At the same time, Argonne National Laboratories continues to conduct computer simulations of this battery reaction in order to further understand the reaction mechanism, in order to enhance the performance of the battery in the future, to provide theoretical support for possible commercialization in the future.
It should be pointed out that although this research is far from commercial applications and its energy density is not far from optimal, it is undoubtedly a major advance in the development of lithium-air batteries.
The results of this research prove that lithium-air batteries can indeed shield other gases from interference, and directly obtain oxygen from air-like atmospheres, get rid of dependence on oxygen storage devices, and have a long cycle life. This undoubtedly greatly enhances the confidence of researchers and industry in the future development of this revolutionary battery technology:
Since the most important problems have clear solutions, the rest may not be fatal at all! It may not take long for researchers to create new batteries with energy densities much higher than existing lithium-ion battery technologies, which will undoubtedly radically change the existing energy landscape.
Magnesium Chloride Hexahydrate, molecular formula is MgCl2.6H2O, EC code is E511. It is a very common food additive, and Food Grade Magnesium Chloride is widely used in food industry (food processing etc.) and health-care products. It is white fine crystalline powder and it load 20MTs/20'GP without pallet.
Magnesium Chloride,Magnesium Chloride Hexahydrate,MgCl2.6H2O,Food Grade Magnesium Chloride,
Jiangsu Kolod Food Ingredients Co., Ltd. , https://www.kolodchem.com